Q: Is Horizon® 90 PT approved for chlorination of drinking water?
Horizon® 90 PT (active ingredient Trichlor-s-triazinetrione) is EPA registered for disinfection of drinking water in public and individual water systems. The US EPA Registration number is 69681-22.
Q: Is Horizon® 90 PT NSF certified?
Horizon® 90 PT is NSF Standard 60 (Drinking Water Chemicals - Health Effects) certified. NSF Certifications are updated daily. Current listings can be viewed on their website as http://www.nsf.org/Certified/PwsChemicals/Listings.asp?Company=4C890&Standard=060
Q: What is the active ingredient in Horizon® 90 PT?
The active ingredient in Horizon® 90 PT is Trichloro-s-triazinetrione. This active ingredient is commonly referred to as "Trichlor", "Trichloro", or "Trichloroisocyanurate". It is one of a family of chemical compounds commonly referred to as chlorinated isocyanurates.
Trichlor is a solid product, typically in a compressed tablet form, that is a common chlorinating and bleaching compound. Similar to other chlorinating compounds like sodium hypochlorite or calcium hypochlorite, Trichlor hydrolyzes in water to form hypochlorous acid (free chlorine) as seen in the equation below:
Trichloroisocyanurate plus Water yields Hypochlorous Acid and Cyanuric Acid
Cl3(NCO)3 + 3 H2O --> 3 HOCl + C3N3O3H3
The resulting HOCl is free to disinfect as well as oxidize a wide variety of organic and inorganic materials. The cyanuric acid resulting from the liberation of HOCl is stable and nonreactive by comparison. In the environment, cyanuric acid is found naturally in soils ranging from 1 to 6 ppm and is rapidly biodegraded under a wide variety of natural conditions. Natural degradation is primarily a hydrolysis process resulting in CO2 and ammonia and no BOD (biological oxygen demand).
Various Chemical and Physical Properties of Trichlor can be found in the following table:
| Chemical Name | Trichloro-s-triazinetrione | ||
| Common Names | Trichloroisocyanurate; Trichlor; Trichloro | ||
| Molecular Weight (g) | 232.41 | ||
| CAS Number | 87-90-1 | ||
| Formula | Cl3(NCO)3 | ||
| Chemical Structure |
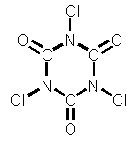 |
||
| Available Chlorine Content | Theoretical = 91.5% | ||
| Minimum 90% | |||
| Color | White | ||
| Appearance | Crystalline powder, granular; tablet | ||
| Melting Point | 225 to 230°C | ||
| Solubility in water (g/100g H2O) | 1.2 at 25°C | ||
| Solubility in Acetone (g/100g Acetone) | 35 at 30°C | ||
| pH, 1% solution | 2.8 at 25°C | ||
| HMIS Ratings | NFPA Ratings | ||
| Health | 3 | Health | 2 |
| Flammability | 0 | Flammability | 0 |
| Reactivity | 2 | Reactivity | 2 |
| Other | Oxidizer (Class 1) | ||
Q: How does Trichlor compare to other forms of chorine?
The amount of chlorine released from all forms of chlorine are compared to gaseous chlorine (elemental chlorine) using the term available chlorine. Elemental chlorine is considered 100% available chlorine. The higher the percentage, the more hypochlorous acid is released from the compound when dissolved in water. Trichlor is 90% available chlorine. It has the highest available chlorine content of any of the solid forms of chlorine.
A higher available chlorine content means less product required to establish and maintain free chlorine residual in the water. Reduced product demand translates to a reduction in the quantity of product handled, transported, stored and applied. the table below describes both the percent available chlorine and the trichlor equivalent in pounds of common chlorinating compounds.
| Qty | Compound | Trichlor | Equivalent |
| 1 lb | Gas Chlorine (100% available Cl2) | equals | 1.1 lbs. |
| 1 lb | Trichlor (90% available Cl2) | equals | 1.0 lbs |
| 1 lb | Calcium Hypochlorite (65% available Cl2) | equals | 0.7 lbs |
| 1 lb | Sodium Hypochlorite (12% available Cl2) | equals | 0.1 lbs |
| 1 gal. | Sodium Hypochlorite (12% available Cl2) | equals | 1.34 lbs |
| 1 lb | Sodium Hypochlorite (55 available Cl2) | equals | 0.06 lbs |
Horizon® 90 PT is considered a less hazardous alternative to sodium hypochlorite and gas chlorine. This is due to its solid form being less prone to spills and leaks as well as spills being much easier to contain and clean up. In comparison to sodium hypochlorite, the pounds of product transported and stored on site is greatly reduced. There is no need for large storage tanks, secondary containment, or containment dikes to control liquid chlorine spills.
Under proper storage conditions, Trichlor is also much more stable than sodium hypochlorite resulting in less frequent deliveries of product.
Because trichlor is a completely soluble form of chlorine, chemical feed systems require little maintenance compared to liquid or calcium hypochlorite systems. Those feed systems require frequent cleaning and maintenance due to insoluble salts and scale resulting in increased maintenance cost and down time.
Q: Is trichloroisocyanurate listed under California Prop 65?
Thrichloroisocyanurate is NOT listed on the California Proposition 65 list of carcinogens, reproductive toxicants and candidate carcinogens.
Q: Is trichloroisocyanurate safe?
Numerous and extensive toxicity studies have been completed and submitted to support the US EPA registration. The data is jointly owned by the Isocyanurate Industry Ad Hoc Committee. The EPA has made some data available under the High Production Volume (HPV) program. Details on the HPV program as well as registered products can be found at www.epa.gov.